Hydrochloric acid or muriatic acid is a strong inorganic acid, produced from the dissolution of hydrogen chloride gas in water. Initially, this acid was produced from sulfuric acid and table salt in the Renaissance, even in the Middle Ages, and was later used by chemists Glauber, Priestley and Davy in their scientific research.
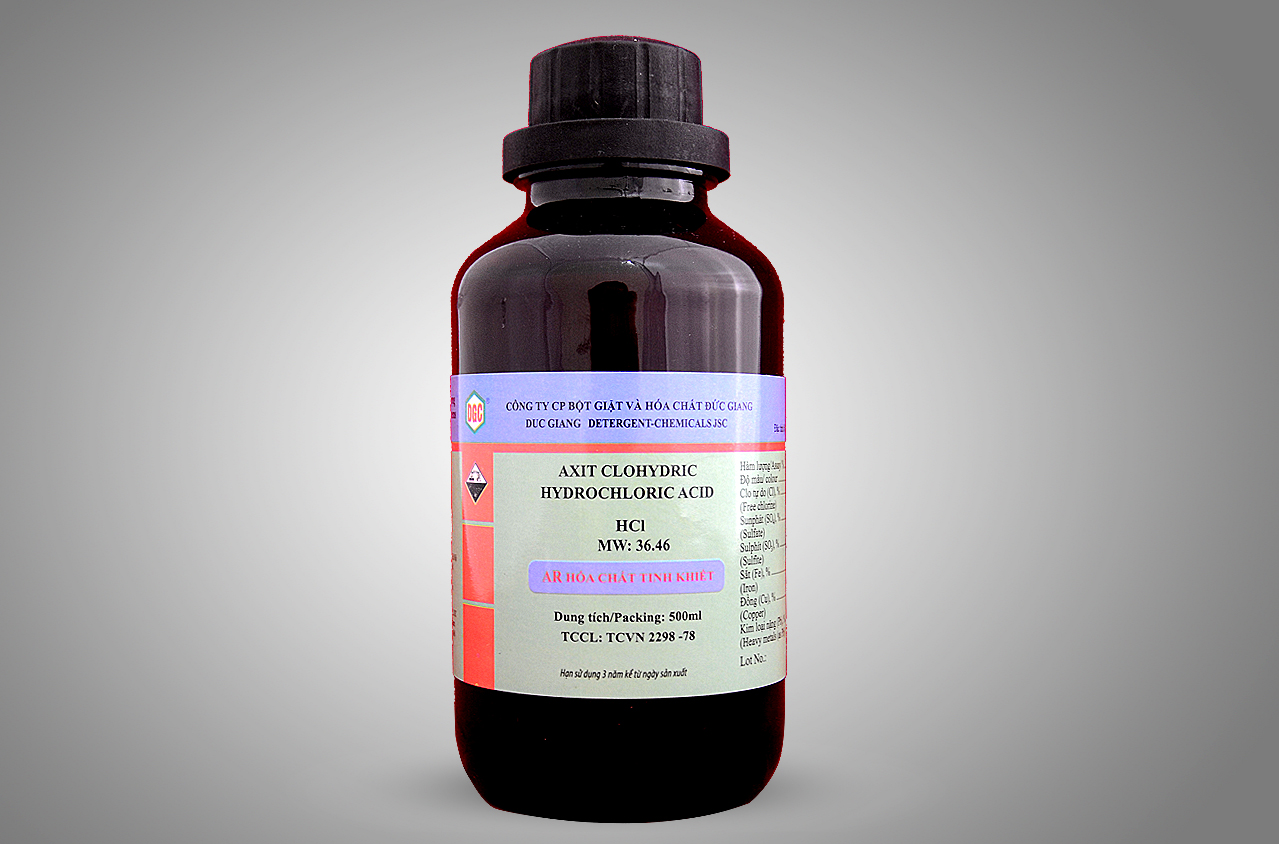
Formula: HCl
Molecular mass: 36.46 g / mol
Classification: Inorganic acid
Water solubility: Soluble limit
Solubility: Soluble in methylene ethers, ethanol, methanol
EU classification: Toxic (T); Causes strong corrosion (C); Dangerous for the environment (N)
| Hydrochloric acid
Axit clohydric |
| HCl |
MW: 36.46 |
Specification
| Item |
Sign |
PA |
P |
| Assay, % ……………………… |
|
36.0~38.0 |
36.0~38.0 |
| Appearance …………………… |
|
Passes test |
Passes test |
| Ignition residue, % …………… |
≤ |
0.001 |
0.002 |
| Free chlorine (Cl), % ………… |
≤ |
0.0001 |
0.0002 |
| Sulfate (SO4), % ……………… |
≤ |
0.0002 |
0.0005 |
| Sulfite (SO3), % ……………… |
≤ |
0.0005 |
0.001 |
| Iron (Fe), % ………………….. |
≤ |
0.00005 |
0.0001 |
| Copper (Cu), % ……………… |
≤ |
0.00001 |
0.0001 |
| Arsenic (As), % ……………… |
≤ |
0.000005 |
0.00001 |
| Tin (Sn), % …………………… |
≤ |
0.0002 |
0.0005 |
| Lead (Pb), % …………………. |
≤ |
0.00002 |
0.00005 |
| UN NO.: 1789 |
R: 34-37 |
| CAS NO.: 7647-01-0 |
S: 26-36/37/39-45 |
| Cat.No. |
Grade |
Quantity |
Packaging |
| DG0115PA0.5L |
PA |
500ml |
Glass Bottle |
| DG0115P0.5L |
P |
500ml |
Glass Bottle |